Introduction: High-grade B cell lymphoma (HGBCL) with rearrangements of MYC in addition to either BCL2 or BCL6 (DHL) generally has a poor prognosis for response to conventional therapies. Nevertheless, current studies of responses to anti-CD19 CAR-T cell therapies (CAR-T) have not demonstrated poorer overall response rates (ORR) in patients with DHL (Locke Lancet Oncol 2019, Schuster NEJM 2019). Double expressor diffuse large B-cell lymphoma (DEL), which lacks the chromosomal rearrangements of DHL but overexpresses MYC and BCL2 proteins by immunohistochemistry, has been similarly associated with poor outcomes, including after autologous and allogeneic stem cell transplantation (Herrera JCO 2017; Kawashima Biol Blood Marrow Transplant, 2018). DEL and DHL each have a different cell of origin and biology; DEL is typically activated B cell-like phenotype, whereas DHL typically have germinal center-like phenotype. To our knowledge, the association of DEL and outcomes with CAR-T has not been assessed. We aimed to assess whether DEL is associated with outcome after CAR-T.
Methods: We retrospectively reviewed the records of 75 consecutive patients with aggressive B-cell lymphomas treated with commercial CAR-T therapy at the University of Pennsylvania between April 2018 and October 2019. We included all patients with either diffuse large B cell lymphoma or HGBCL who had both: a) fluorescence in situ hybridization testing for MYC, BCL2 and BCL6 chromosomal rearrangements, and b) immunohistochemical staining (IHC) for MYC and BCL2. Patients who had both MYC and BCL2 and/or BCL6 chromosomal rearrangements were classified as DHL; patients with IHC expression of both MYC in > 40% and BCL2 in > 50% of tumor cells without meeting criteria for DHL were classified as DEL. Patient characteristics were collected. Fisher's exact test was used to compare overall response rate (ORR) and log-rank test was used for comparison of progression-free survival (PFS).
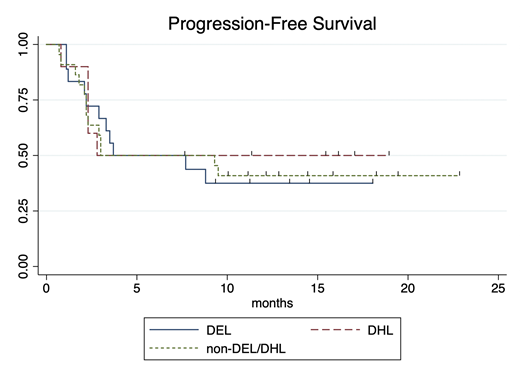
Results: Seventy-five eligible cases of aggressive B-cell lymphoma were identified; 9 were excluded due to non-DLBCL or HGBCL subtype and 16 were excluded due to insufficient characterization of tissue to assess DHL and DEL status. Fifty patients were eligible for analysis; 18 patients (36%) met criteria for DEL, 10 patients (20%) met criteria for DHL, and the remaining 44% of patients had neither DEL nor DHL (non-DEL/DHL). Tisagenlecleucel was administered to 39 patients, whereas axicabtagene ciloleucel was administered to 11 patients. Median age at leukapheresis was 65 years (range: 35-81). LDH, administration of bridging therapy, and ECOG performance status (PS) did not significantly differ between the three groups. Median follow-up for the entire cohort was 15.9 months. For all patients, best ORR was 54% (27/50 patients), median PFS was 3.7 months (95%CI: 2.8-NE), and median OS was not reached. Best ORR was 56% (10/18) for DEL patients, 50% (5/10) for DHL patients, and 55% (12/22) for patients with non-DEL/DHL. ORR did not differ between these groups (p=1.0). There was no difference in PFS (see Figure, p=0.90), OS (p=0.61), or RD (p=0.38) between the three groups. At 16 months, 69% of responding DEL continue in CR (95%CI: 30-89%), 72% of non-DEL/DHL (95%CI: 35-90%) continue in CR, and 100% of DHL (95%CI: NE) continue in CR.
Conclusions: The proportion of patients with DEL in our study was similar to that reported for patients with diffuse large B-cell lymphoma in the literature. There were no significant differences between DEL, DHL, and non-DEL/DHL groups with regard to the proportion of patients with elevated LDH, bridging therapy, or ECOG PS. Although DHL and DEL have a negative prognostic impact on response to salvage therapies and hematopoietic stem cell transplant, neither DHL nor DEL status was associated with inferior response to anti-CD19 commercial CAR-T products. We demonstrate that patients with DEL can achieve prolonged responses after CAR-T. Moreover, neither DEL nor DHL status appeared to impact PFS, OS, or RD outcomes after commercial CAR-T.
Chong:BMS: Membership on an entity's Board of Directors or advisory committees; Tessa: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; KITE Pharma: Membership on an entity's Board of Directors or advisory committees. Landsburg:Curis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Triphase: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Speakers Bureau; Takeda: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees. Gerson:Loxo: Research Funding; Genentech: Consultancy; Pharmacyclics: Consultancy; Abbvie: Consultancy. Svoboda:Atara: Consultancy; Genmab: Consultancy; TG: Research Funding; Adaptive: Consultancy; Astra-Zeneca: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Imbrium: Consultancy; Seattle Genetics: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding; Incyte: Research Funding. Dwivedy Nasta:Merck: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Debiopharm: Research Funding; Forty Seven: Research Funding; Incyte: Research Funding; Atara: Research Funding; Rafael Pharmaceuticals: Research Funding. Barta:Atara: Honoraria; Pfizer: Honoraria; Janssen: Honoraria; Seattle Genetics: Honoraria, Research Funding; Monsanto: Consultancy. Garfall:Novartis: Research Funding; Tmunity: Consultancy, Other: Personal fees, Research Funding; Amgen: Research Funding; Kite Pharma: Other: Personal fees; Janssen: Consultancy, Research Funding; GSK: Consultancy; Surface Oncology: Consultancy. Ruella:UPenn/Novartis: Patents & Royalties; Abclon, BMS, NanoString: Consultancy; Abclon: Consultancy, Research Funding. Frey:Amgen: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Syntax: Consultancy, Honoraria. Porter:Incyte: Other: Advisory board; Novartis: Honoraria, Other: Advisory board, Patents & Royalties: CAR T cells for CD19+ malignancies, Research Funding; Janssen: Other: Advisory board; Genentech/Roche: Current equity holder in publicly-traded company, Other: Spouse employment (ended Sept 2020); her salary includes stock/options; Glenmark: Other: Advisory board; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; American Board of Internal Medicine: Other: Member, exam writing committee (end date Oct 2019); Tmunity: Patents & Royalties; Adicet bio: Other: Advisory board; Kite/Gilead: Other: Advisory board. Schuster:AlloGene, AstraZeneca, BeiGene, Genentech, Inc./ F. Hoffmann-La Roche, Juno/Celgene, Loxo Oncology, Nordic Nanovector, Novartis, Tessa Therapeutics: Consultancy, Honoraria; Novartis, Genentech, Inc./ F. Hoffmann-La Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal